Both vaccine candidates incorporate Novavax proprietary. NanoFlu its quadrivalent influenza nanoparticle vaccine met all primary objectives in its pivotal Phase 3 clinical trial in older adults and will be advanced for regulatory submission.
J J Seeks Permission For Phase 3 Trial Of Its Single Shot Covid Vaccine In India Import Licence Times Of India
After a successful Phase 3 trial vaccine manufacturers submit an application to regulatory bodies such as the European Commission or the US.

Phase 3 clinical trial vaccine. PARIS and LONDON May 27 2021 Today Sanofi and GSK started enrollment in their Phase 3 clinical study to assess the safety efficacy and immunogenicity of their adjuvanted recombinant-protein COVID-19 vaccine candidate. The vaccine known as mRNA-1273 was co-developed by the Cambridge Massachusetts-based biotechnology company Moderna Inc and the National Institute of Allergy and Infectious Diseases. While safety remains a focus these trials are primarily about showing that people that have received.
That is below the rates of the shots from Moderna Inc. The Phase 3 trial is designed as a 11 vaccine candidate to placebo randomized observer-blinded study to obtain safety immune response and efficacy data needed for regulatory review. The Phase 3 trials on more than 28000 volunteers across 50 centers showed an efficacy rate of 67 Cadila said.
Novavax is conducting late-stage clinical trials for NVX-CoV2373 its vaccine candidate against SARS-CoV-2 the virus that causes COVID-19. The Coronavirus Efficacy COVE phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV. These findings supported progression of the BNT162b2 vaccine candidate into phase 3.
Food and Drug Administration FDA. One is that although phase 1 and 2 trials establish safety they dont tell the whole story. Phase 3 trials are the pivotal final trials before a vaccine is approved for widespread use.
Savannah Georgia CNN The first Phase 3 clinical trial of a coronavirus vaccine in the United States began Monday. First DNA Covid Vaccine Found 67 Effective in Clinical Trials. A booster study programme will begin in the coming weeks to complement the Phase 3 trial Pending positive Phase 3 outcomes and regulatory reviews the vaccine could be approved in Q4 2021 Today Sanofi and GlaxoSmithKline plc GSK started enrolment in their Phase 3 clinical study to assess the safety efficacy and immunogenicity of their adjuvanted recombinant-protein COVID-19.
Phase 3 also looks at safety and because many more subjects are involved Phase 3 can identify. Cadila based in Ahmedabad Gujarat said the study was carried out during the peak of. The global randomized double-blind placebo-controlled Phase 3 study will include more than 35000 volunteers aged 18 and older from several countries including.
The FDA guidance for Emergency Use Authorization suggests a median duration of follow-up of phase 3 vaccine trial volunteers of 2 months 43. A multi-site Phase 3 clinical trial evaluating an investigational COVID-19 vaccine known as AZD1222 has begun. The trial will enroll approximately 30000 adult volunteers at 80 sites in the United States to evaluate if the candidate vaccine can prevent symptomatic coronavirus disease 2019 COVID-19.
Here we report safety and efficacy findings from the phase 23 part of a global phase 123 trial. At this stage clinical trial data is reviewed to make sure the vaccine is safe and effective. We have already seen the headline results for this vaccine over the past few weeks.
The results of the phase 3 clinical trials of the PfizerBioNTech COVID-19 vaccine candidate have been published in NEJM. A Phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 COVID-19 in adults has begun. The Phase 3 clinical trial of BNT162b2 began on July 27 and has enrolled 43661 participants to date 41135 of whom have received a second dose of the vaccine candidate as of November 13 2020.
The US has a fourth of global. Dr Julian Tang Honorary Associate ProfessorClinical Virologist University of Leicester said. And Pfizer-BioNTech that use messenger RNA technology.
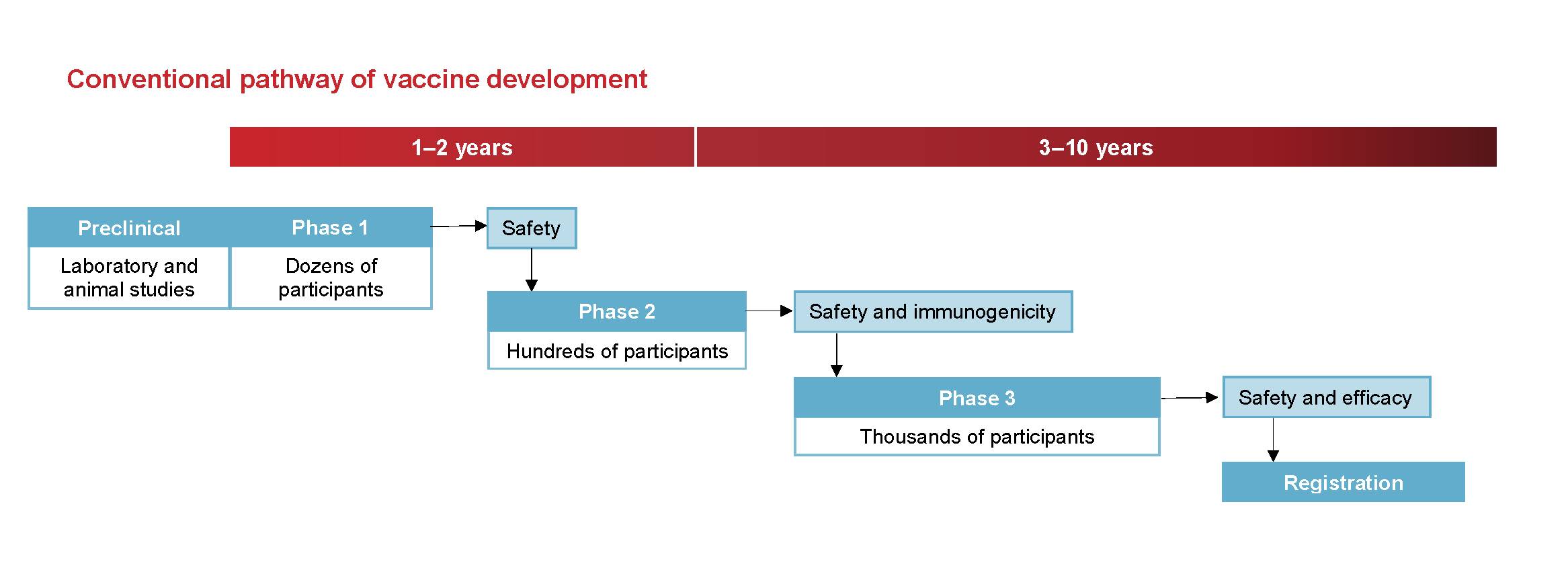
Phases Of Clinical Trials Ncirs
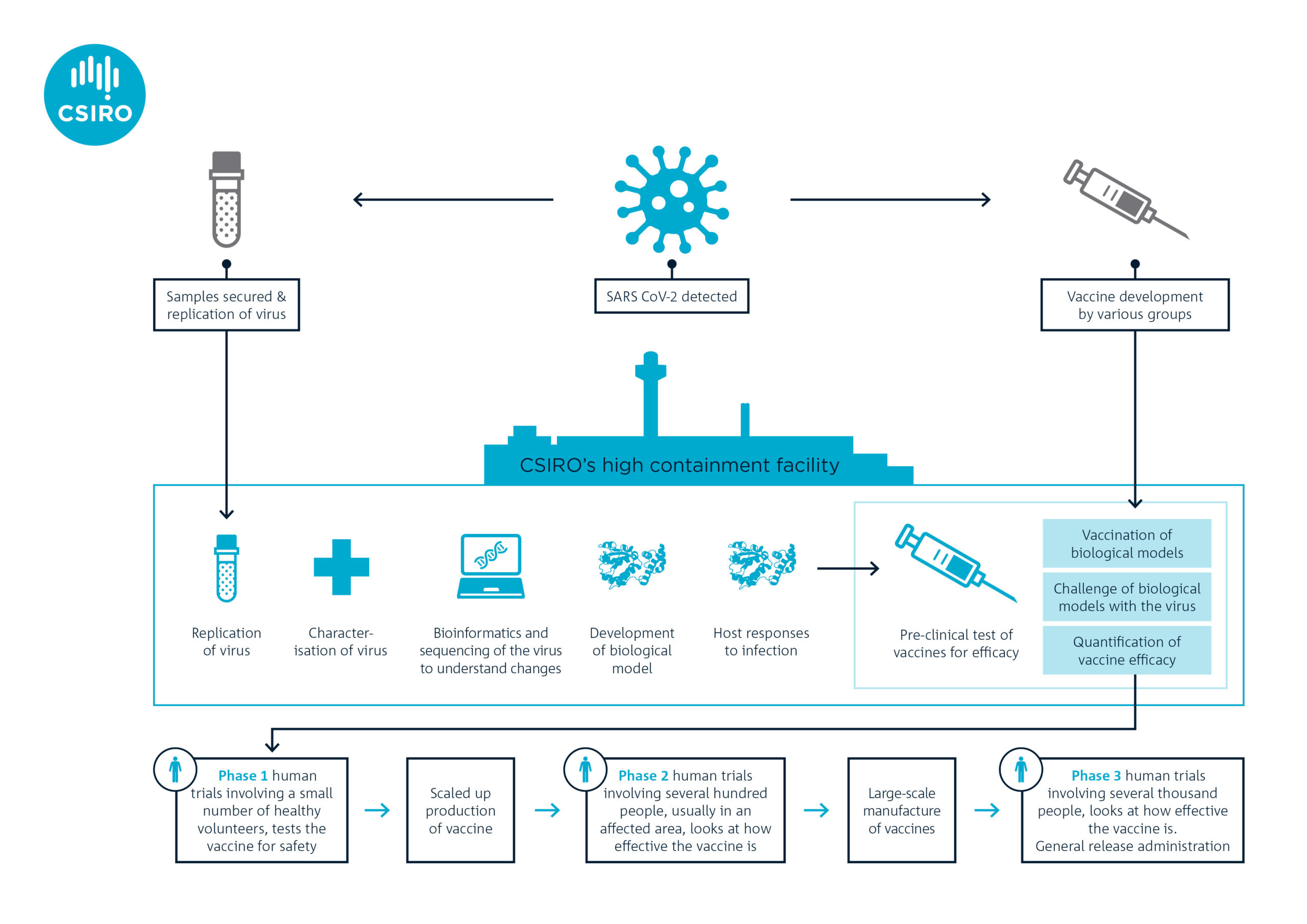
Pre Clinical Covid 19 Vaccine Trials Begin At Csiro Csiroscope
3 000 Volunteers In Phase 3 Clinical Trial For Covid 19 Vaccine Pm Muhyiddin
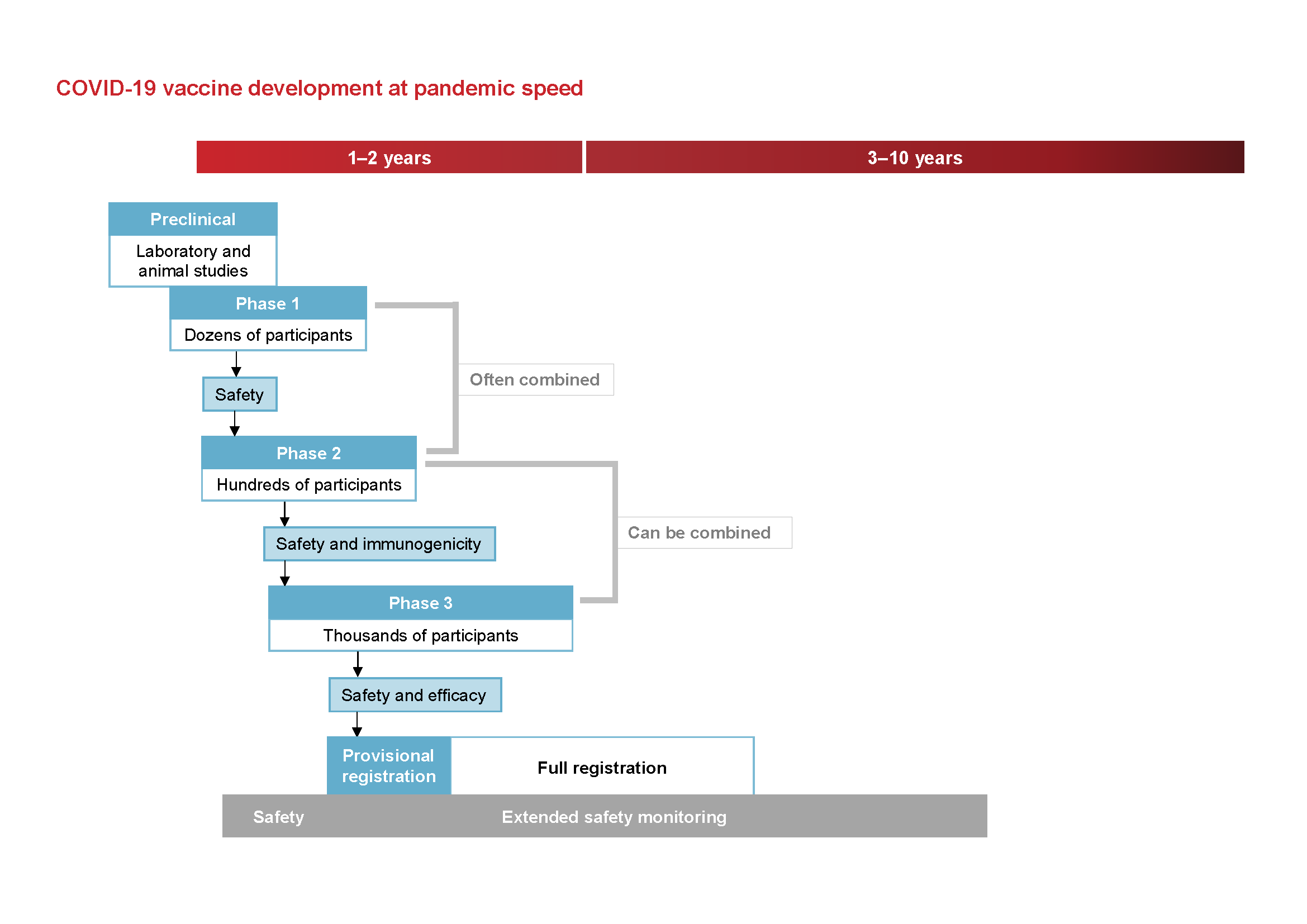
Phases Of Clinical Trials Ncirs

The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson

The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson

Understanding Clinical Trial Terminology What S A Phase 1 2 Or 3 Clinical Trial Concert Pharmaceuticals

Covid 19 Vaccines November Update Progress Of Clinical Trials Post

Us Firm Novavax Partners With Serum Offers 1 1 Bn Covid 19 Vaccine To Covax

Pfizer Biontech S Covid 19 Vaccine Shows High Efficacy In Phase Iii Study
Uae To Become Middle East S Vaccine Center With China S Contribution Global Times
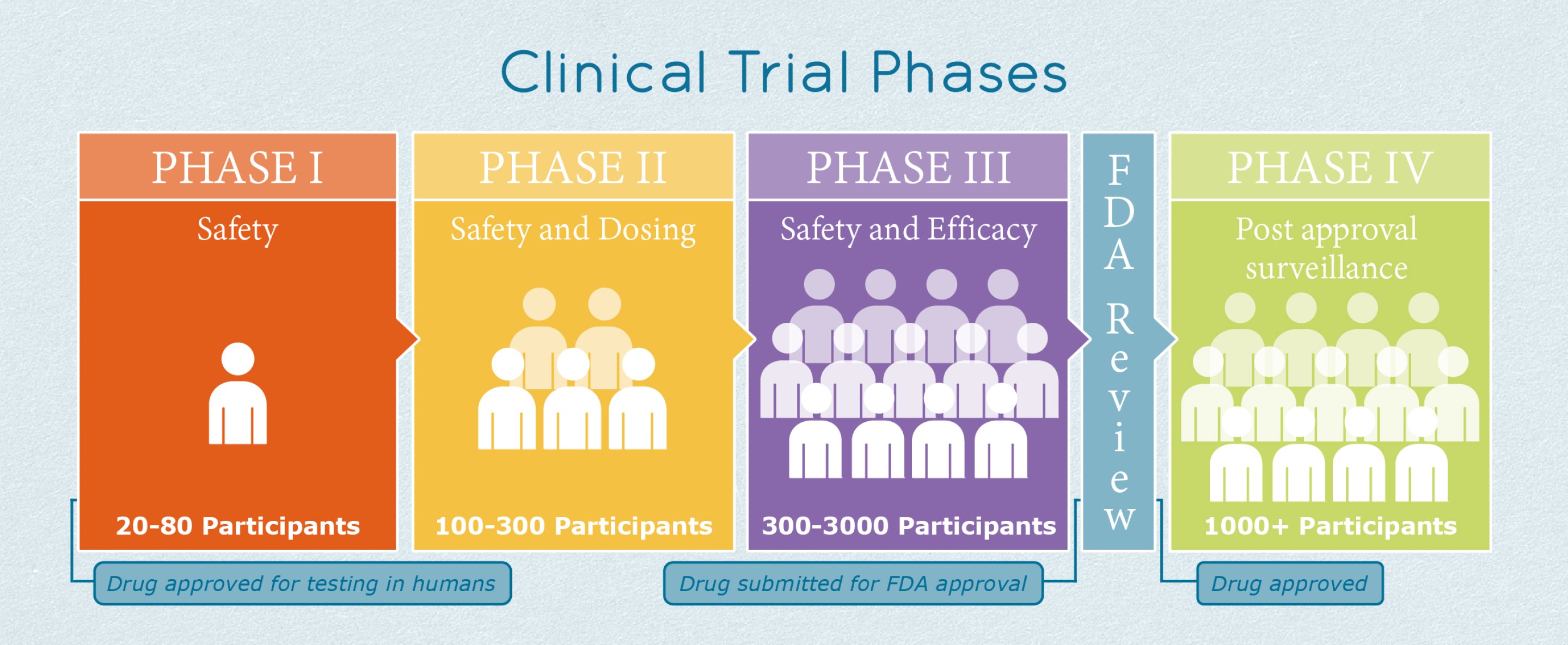
Phase 3 Ipf Clinical Trials Ild Collaborative

Sinovac Reports Positive Data From Phase I Ii Trials Of Coronavac
Covovax Phase 3 Trial To Proceed Without Placebo As Dcgi Gives Nod To Revised Protocol Pune News
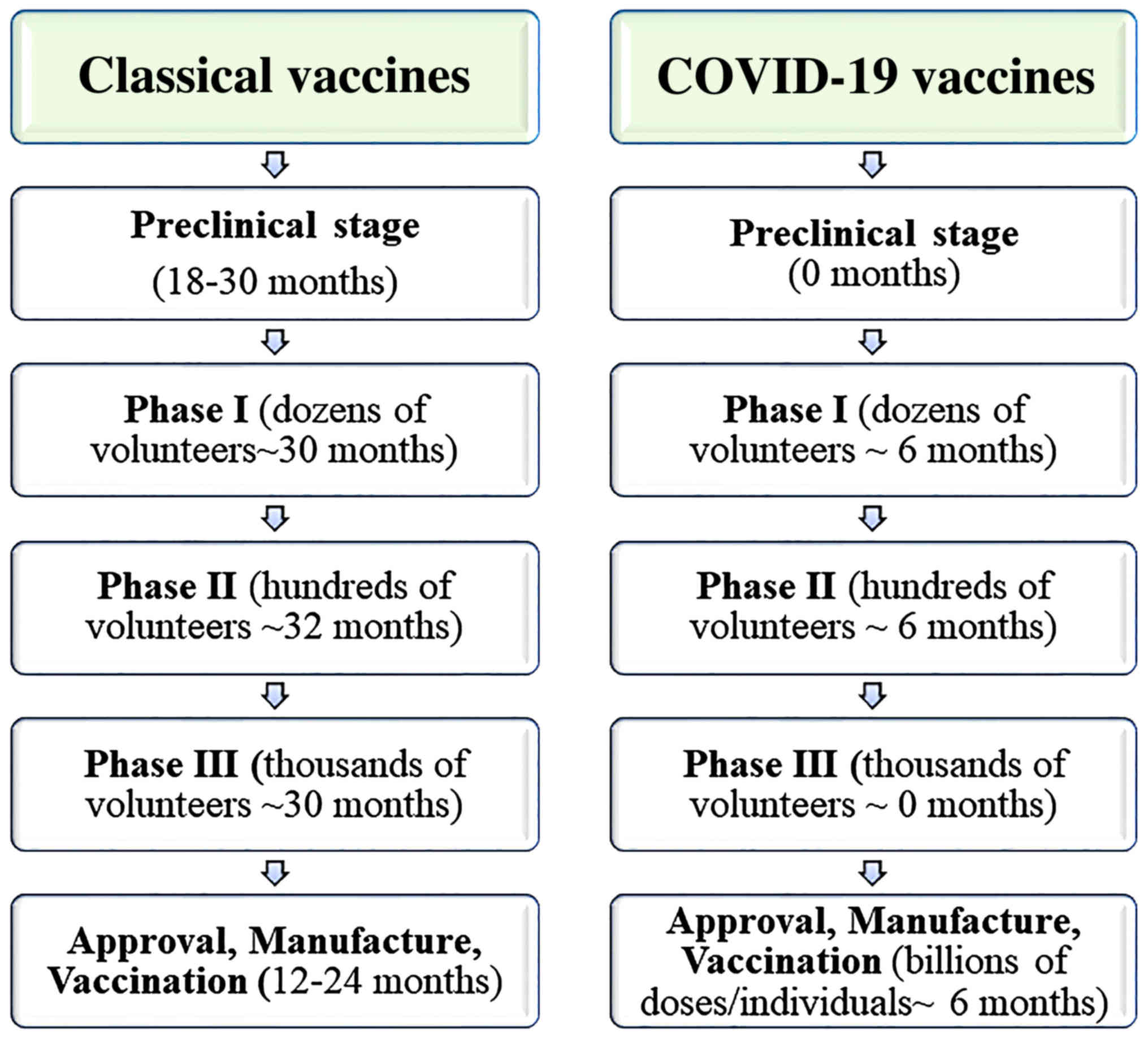
Towards Effective Covid 19 Vaccines Updates Perspectives And Challenges Review

China Begins Phase I Clinical Trial Of Covid 19 Vaccine
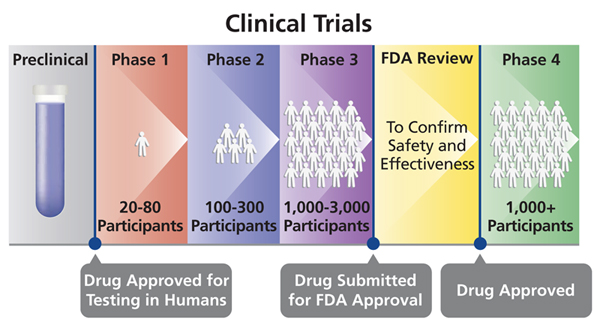
First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
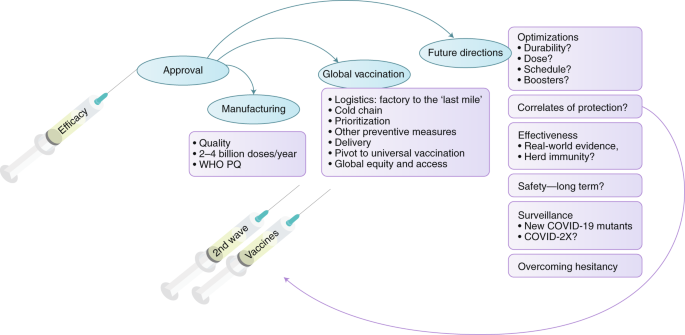
Looking Beyond Covid 19 Vaccine Phase 3 Trials Nature Medicine

Safety And Immunogenicity Evaluation Of Recombinant Bcg Vaccine Against Respiratory Syncytial Virus In A Randomized Double Blind Placebo Controlled Phase I Clinical Trial Eclinicalmedicine
